Background
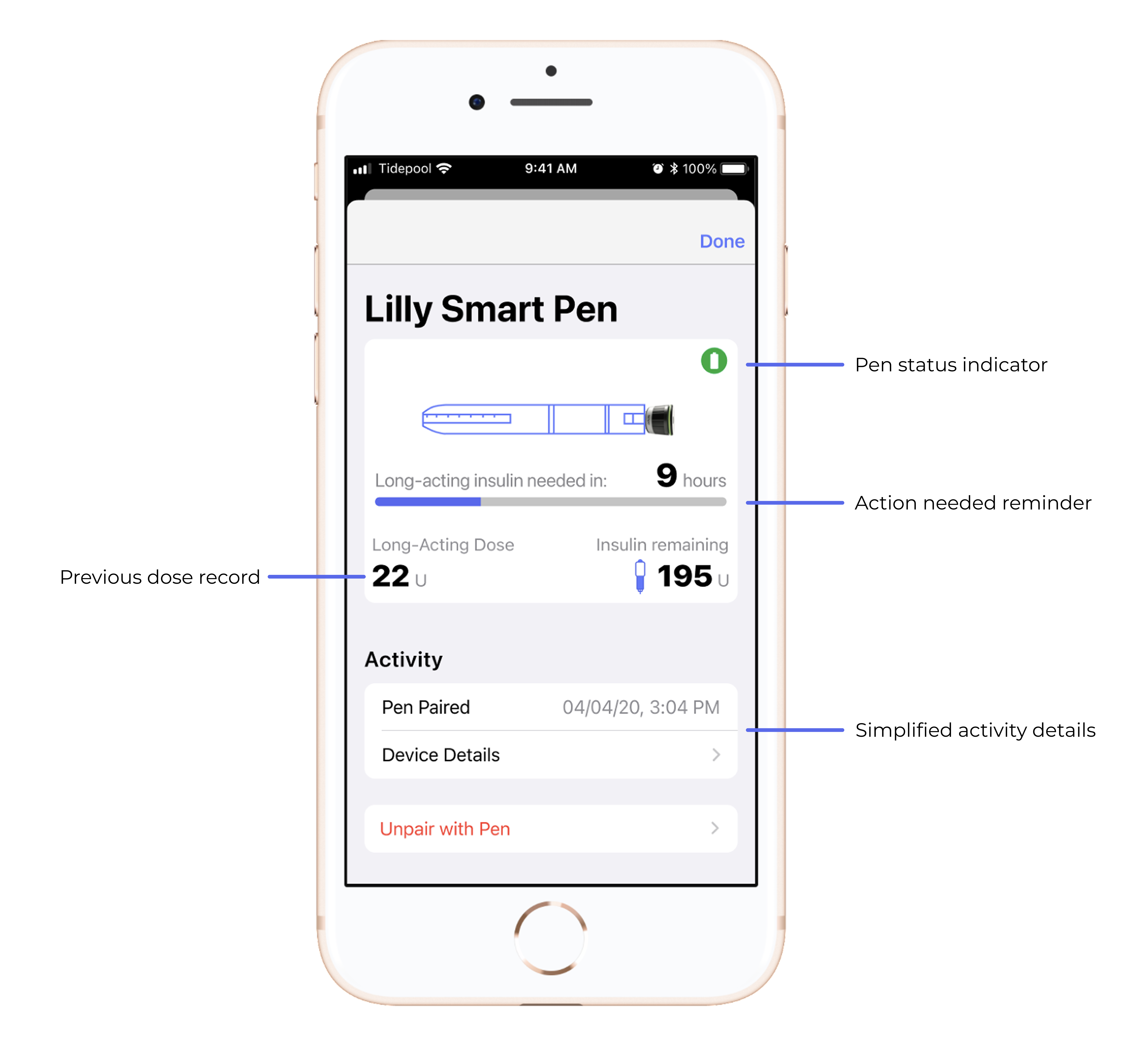
Tidepool Loop
DIY Loop began as a community driven project as part of the #WeAreNotWaiting movement for people with Type 1 diabetes to gain control over their care. Tidepool further developed Loop and to bring it through the FDA process and broaden access beyond its technologically-advanced, original user base. Loop is an iAGC (Interoperable Automated Glycemic Controller) iOS app, connecting between a hardware CGM (Continuous Glucose Monitor) and insulin pump to form an AID (Automated Insulin Delivery system).
Several Challenges
Since the product began as a grassroots effort, a lot of open questions remained about the safety and usability of Loop in the general intended use population. I led the following projects relating to Tidepool Loop's regulatory approval process and subsequent integration with Tidepool's existing suite of products and our hardware partner companies:
- Human factors redesign
- Device Integrations
- Tidepool Web Clinic admin research
- Tidepool Loop blue sky ideation
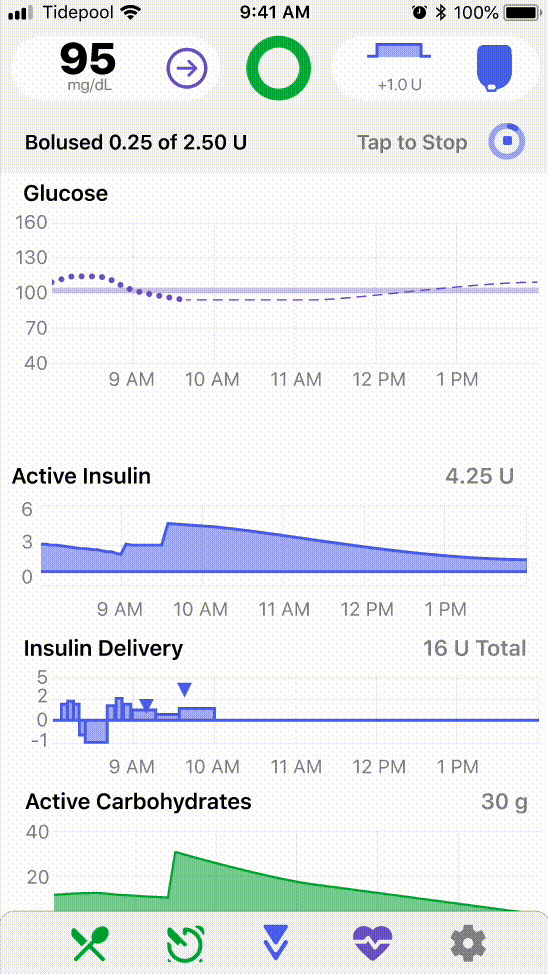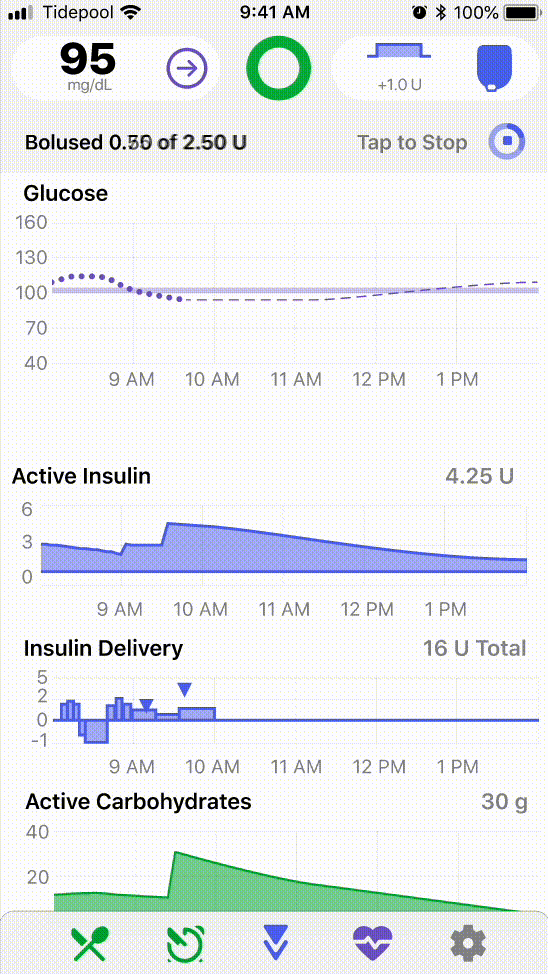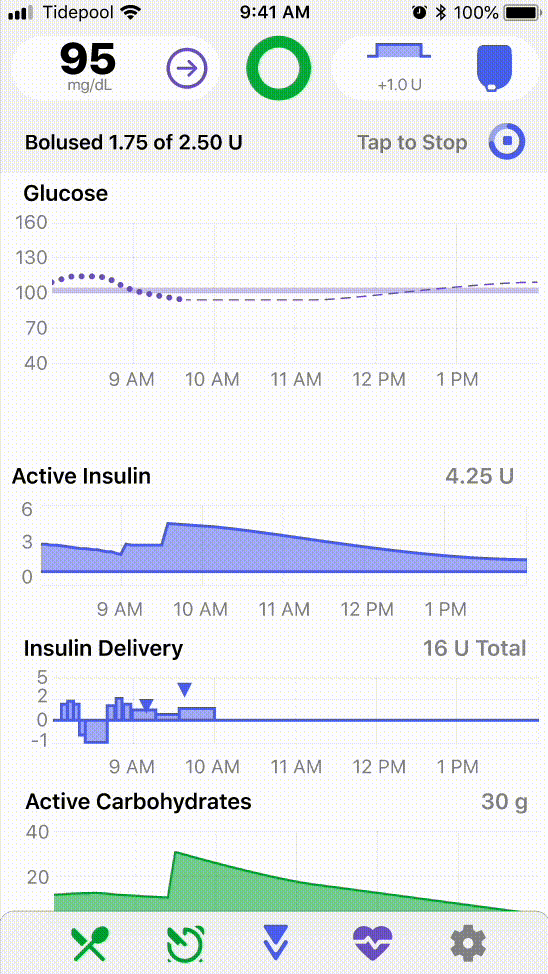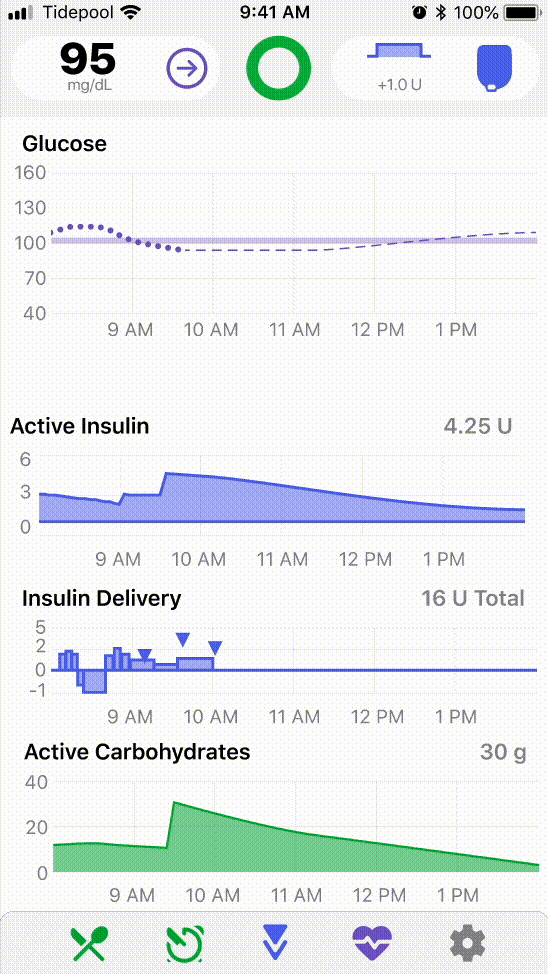
1. Human Factors Redesign
I led the overall strategy, usability testing, prototyping, and data analysis of Tidepool’s inaugural human factors study with a team of two product designers and a product manager, carefully balancing regulatory precedent from our clinical study and development scope changes with the needs of our users.
Design Outcomes
After each round of usability testing, I analyzed task performance and prioritized features that needed further iterations. Design jam sessions were held to brainstorm mitigations, after which I worked with design to refine and development to implement. Some UX changes I led that resulted in significant usability improvements touched features including:
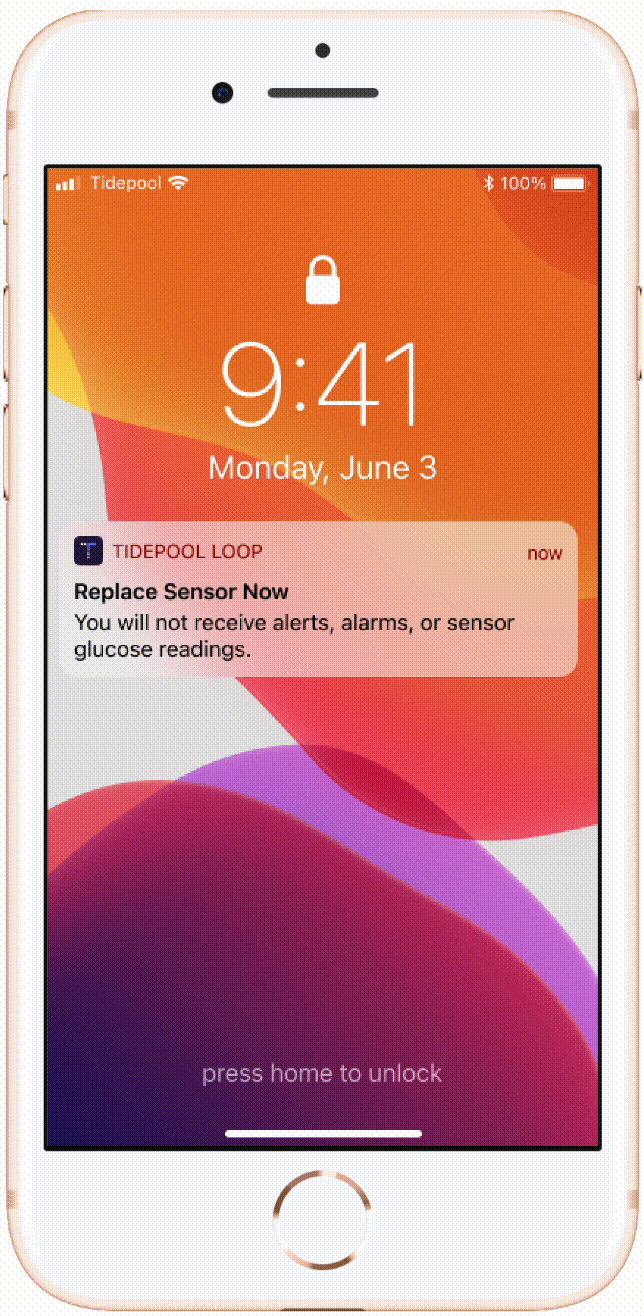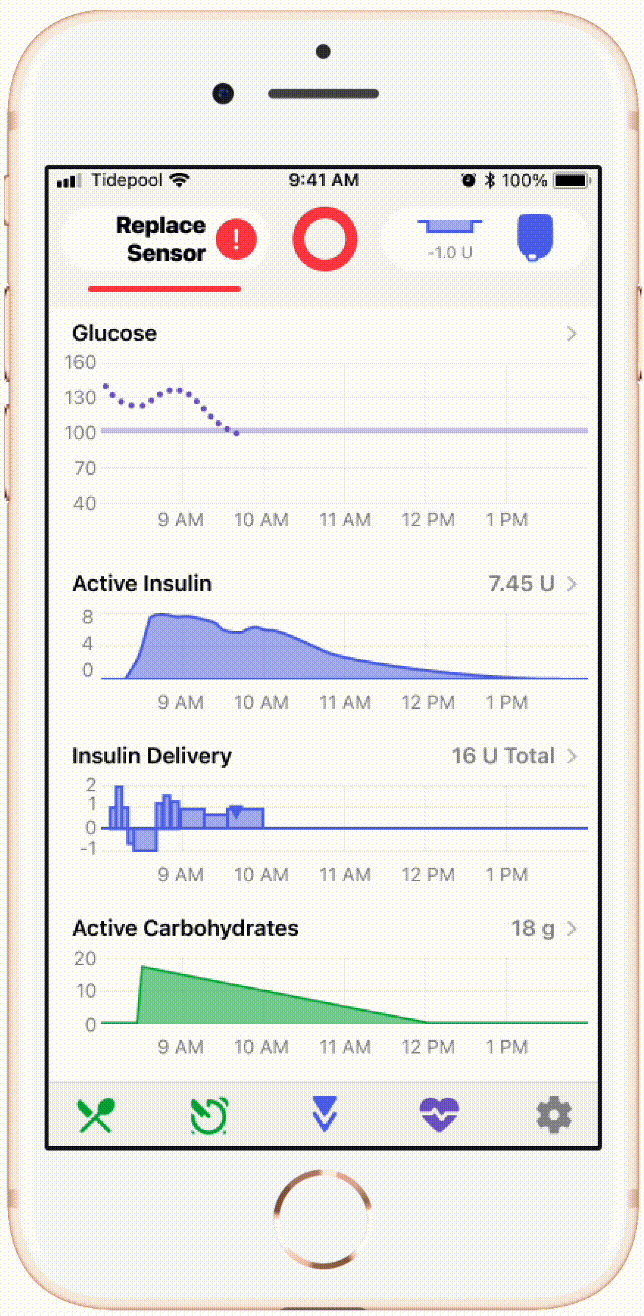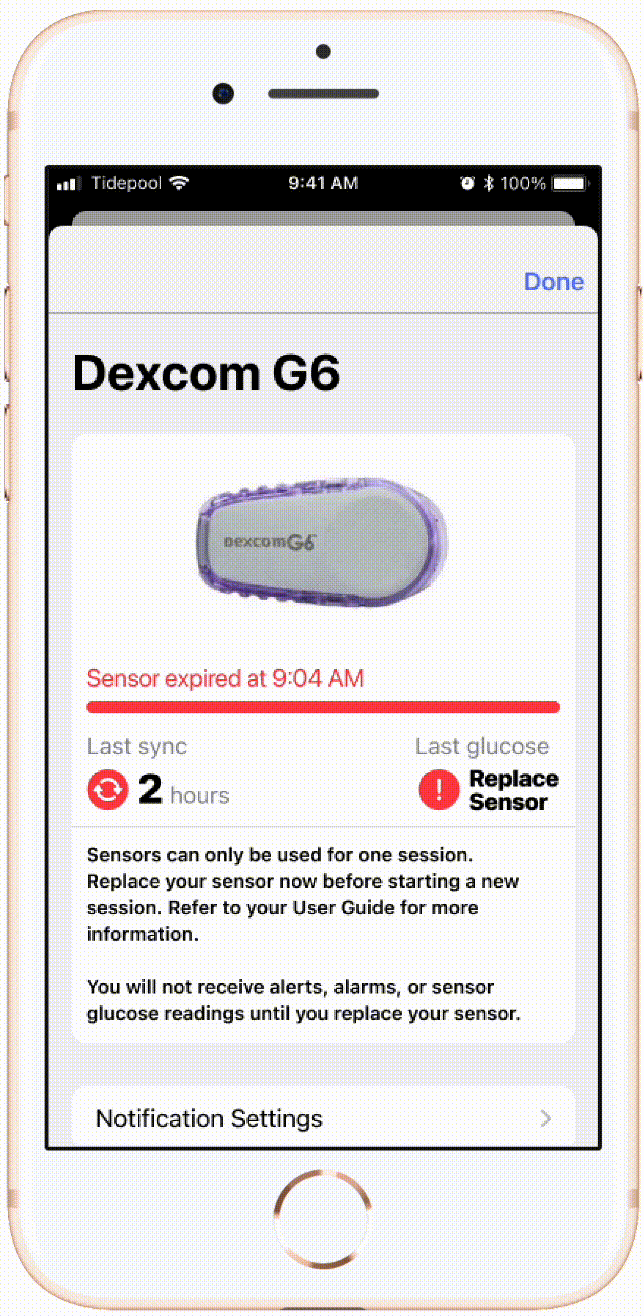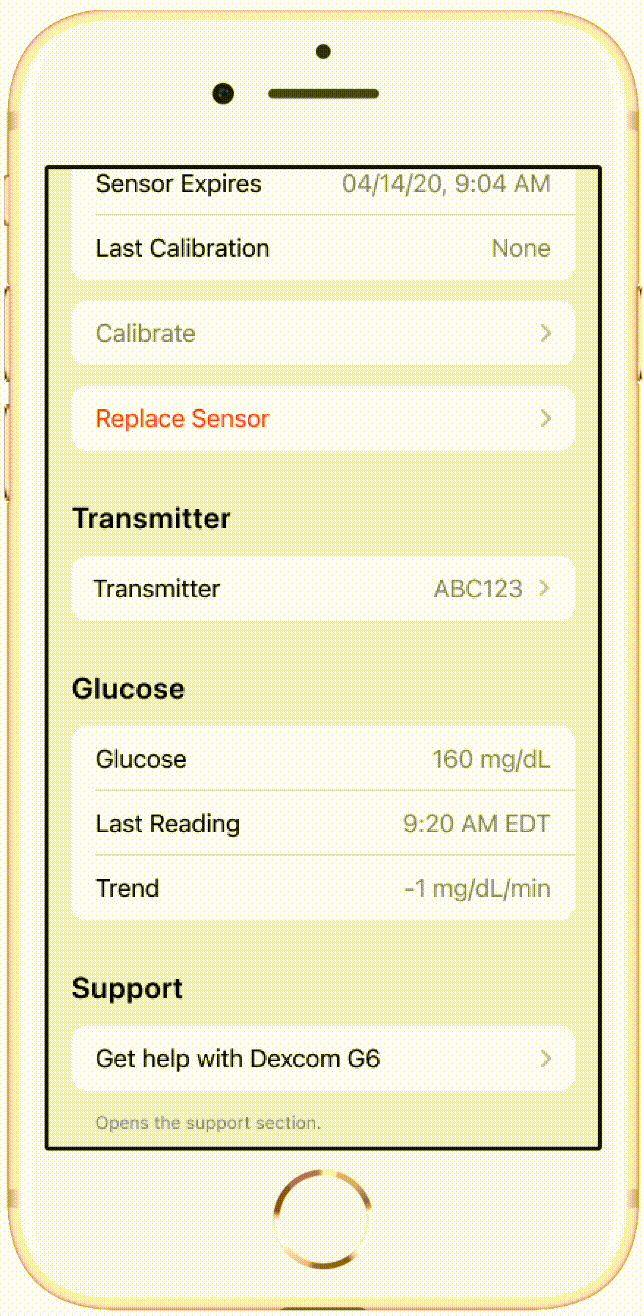
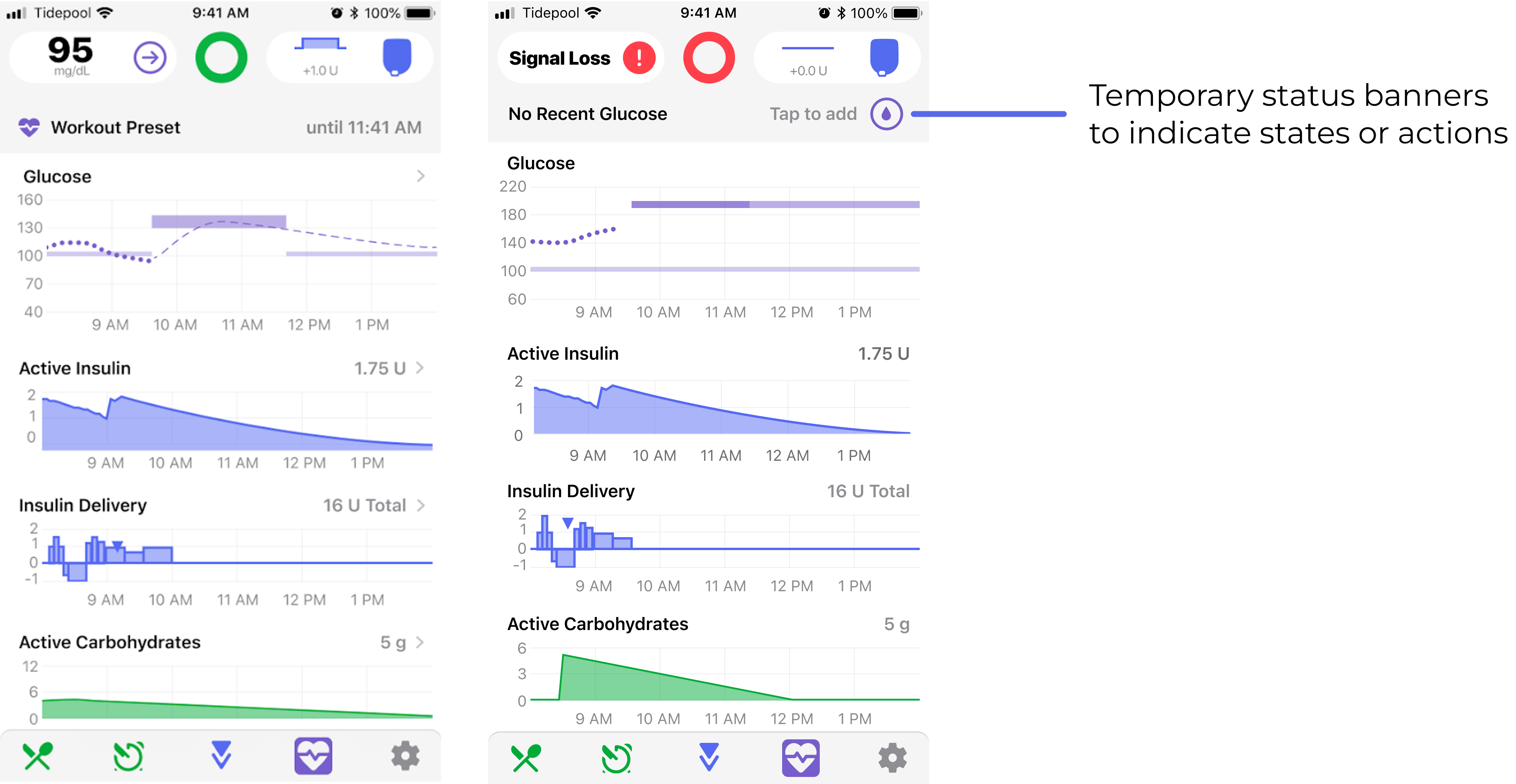
Temporary status banners
Calls to action in these banners helped users with issues noticing certain states and taking actions based on that information.
.png)
Pre-meal and Workout glucose target Presets
Additional actions and indications helped prevent users from unintentionally enabled these presets without understanding.
.png)
Therapy settings safety mechanisms
Copy and UX changes helped users understand their settings guardrails and guard against unintentional changes.
Study Design
Due to the pandemic and also as part of cost saving measures for the nonprofit, I implemented novel remote methods in human factors. This enabled the project to continue at a time when many other medical device companies had paused their efforts. Tidepool had never done human factors before, so I was also responsible for training moderators and leading planning and coordination for the studies.
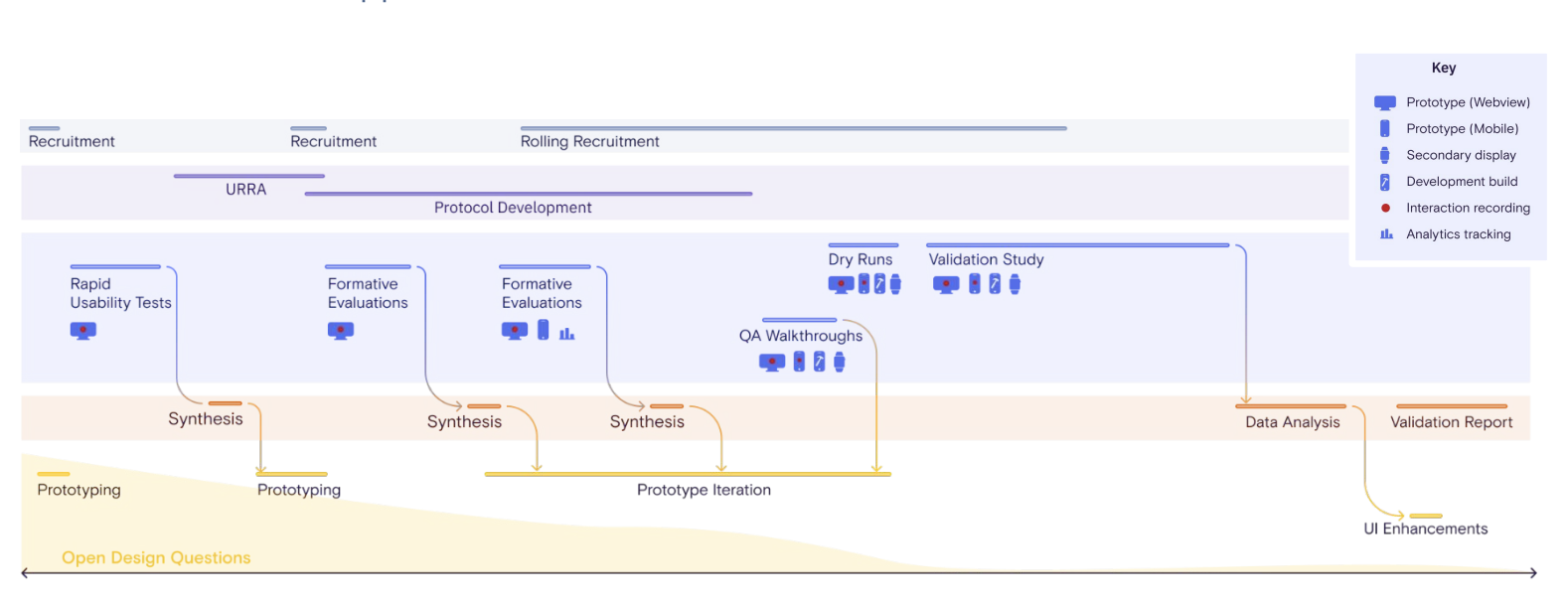
Diagram I designed to capture all aspects of the study from recruitment to risk, devices and tracking, design and development
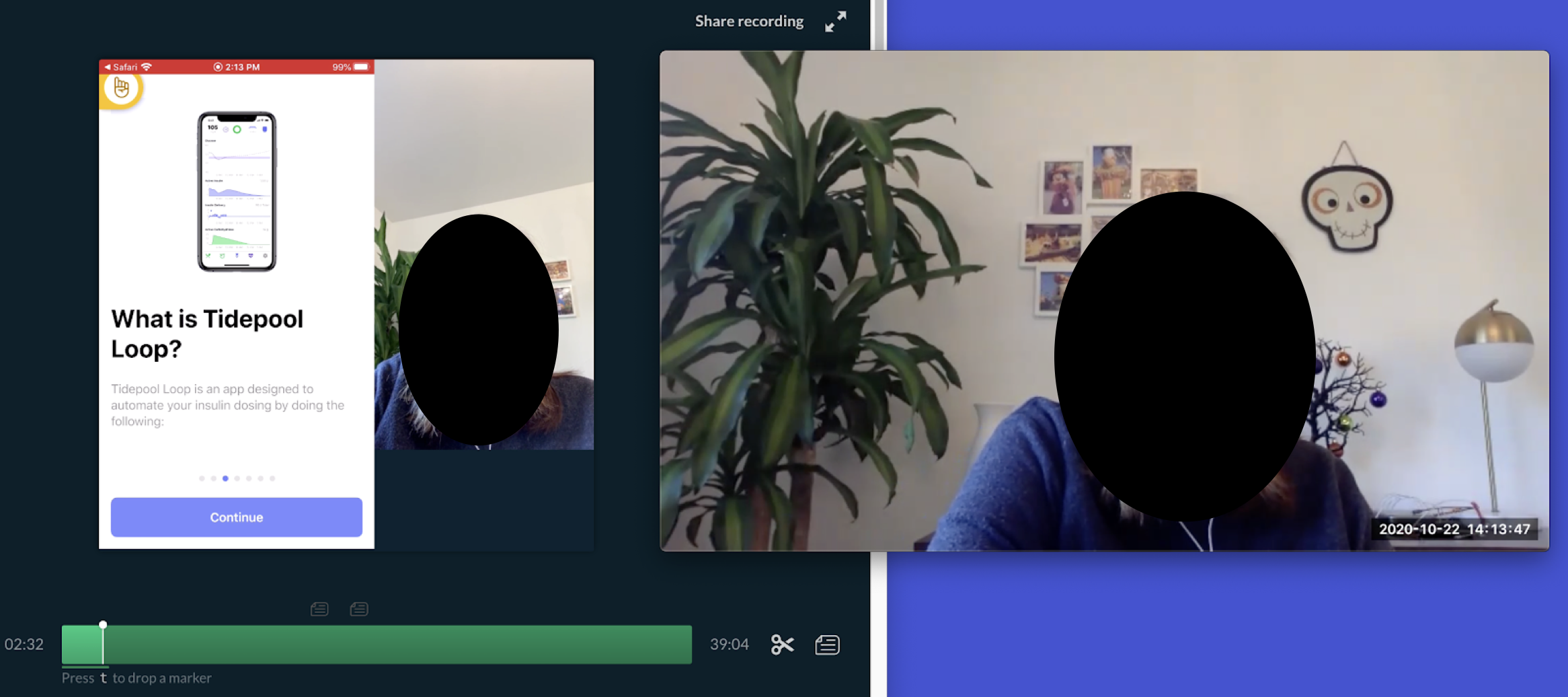
Moderator view of the setup
Research Protocol
The final validation study, with the final protocol and iAGC device submitted to the FDA, included 51 participants of ages, genders, diverse device experiences, diabetes experience, geographic locations, and caregiving responsibility.
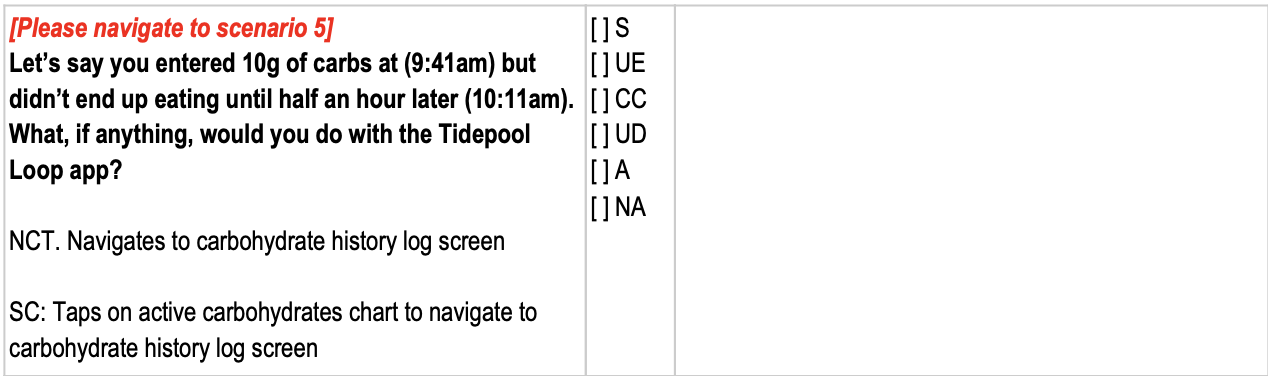
Sample scenario from the moderator's guide
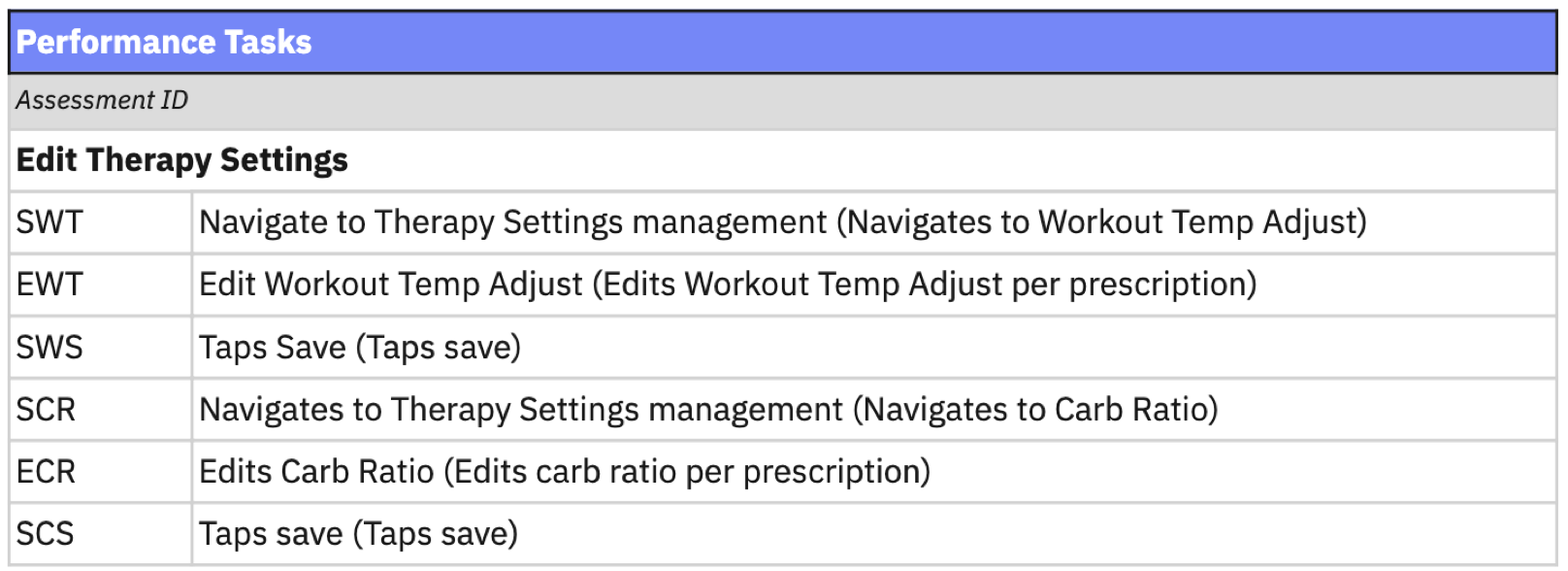
An example of a critical task assessed
The final validation study, with the final protocol and iAGC device submitted to the FDA, included 51 participants of ages, genders, diverse device experiences, diabetes experience, geographic locations, and caregiving responsibility.

An example of the diverse distribution recruited and tested in our pediatric cohort
Participant Feedback
Upon completion of the validation study, I authored the validation study report containing the data analysis results submitted to FDA and assisted with the human factors engineering/usability engineering (HFE/UE) report. Participants who interacted with the product, were overwhelmingly positive, and had some of this to say about Tidepool Loop:
“Tis the magic device”
“Revolutionary”
“Peace of mind”
“It’s come so far. I used to have to pee in a bowl and dip a stick in it.”
2. Device Integrations
Product Development
Central to Tidepool Loop is the concept of interoperability, in which patients can mix and match between devices (insulin pumps and continuous glucose monitors) with Loop serving as the controller, thus freeing up patient choice. With that in mind, I worked with a team of two product designers and two iOS developers to implement the product strategy for our device integrations. I managed the sprint process and facilitated discussions with key stakeholders based on requirements from Tidepool’s device partners and feedback from usability testing.
An example of flow I led implementation of with our CGM device partner company Dexcom
Design for Interoperability
New user facing elements and flows introduced by our device partners with each integration created new scenarios involving critical tasks. I had to develop a strategy for continuous validation of UI enhancements and new features in Tidepool Loop moving forward post-510k submission to the FDA, in line with our commitment to interoperability.
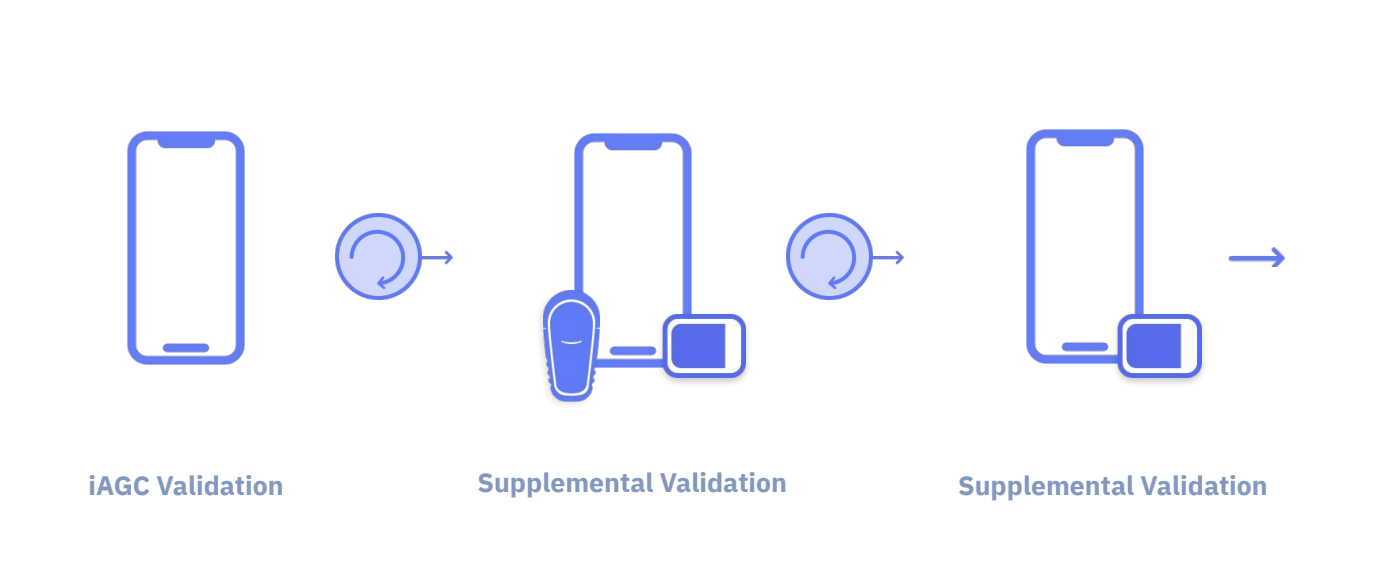
Diagram for regulatory pathway post 510k clearance
Partner Alignment
I facilitated several workshops with industry partners on the developed framework for decentralized, remote human factors research. This work has been presented via poster at the ATTD 2021 conference and published in the Journal of Diabetes Technology & Therapeutics.
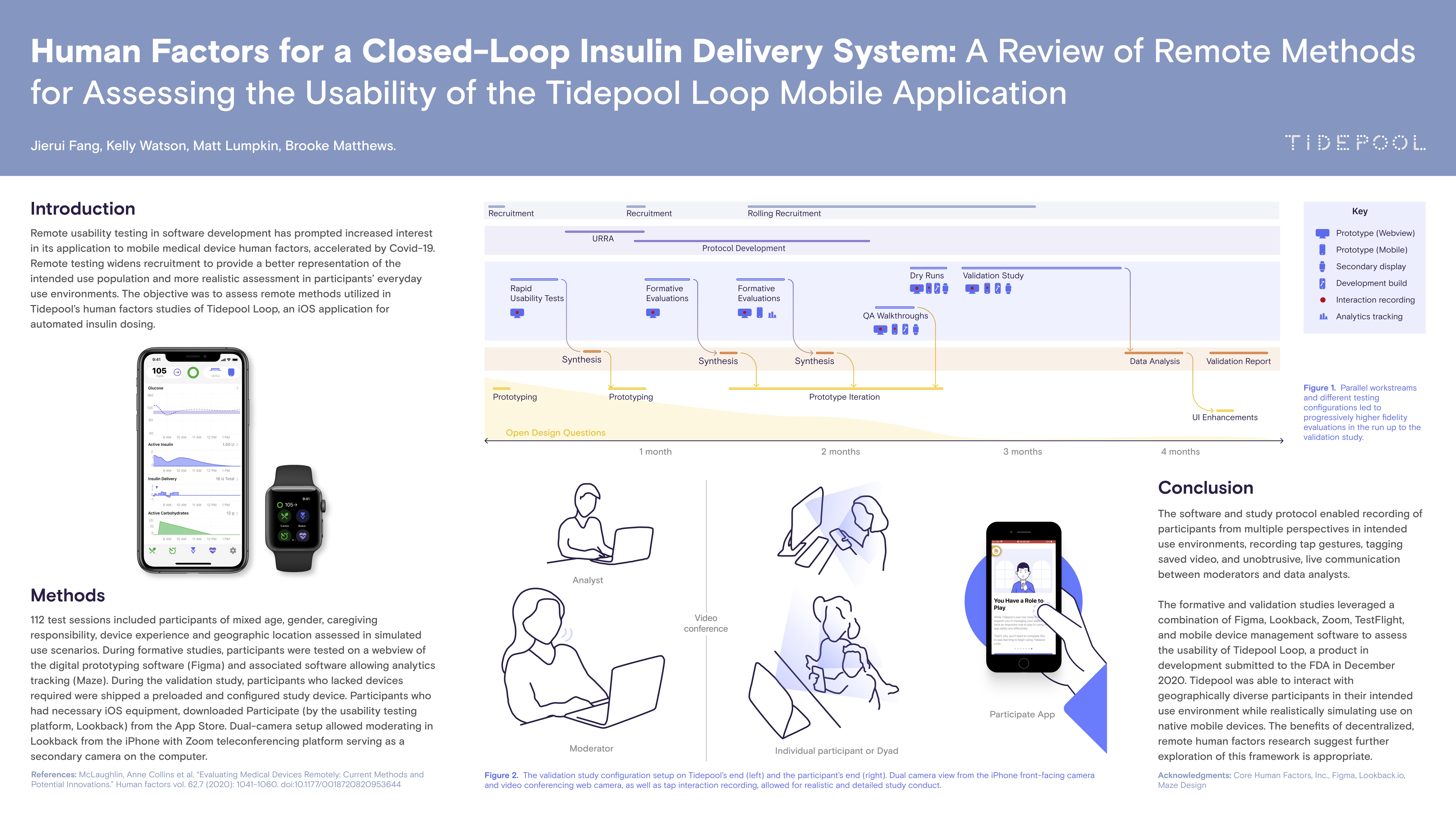
Poster I authored presented at ATTD 2021
3. Clinic Administration
Tidepool Web provides a way for people with diabetes to view their data from a variety of glucose monitoring devices and make treatment decisions with their care team. The account management process however for clinicians is relatively basic and requires clinics with hundreds of clinicians so share one account in order to have the same patient list. With Tidepool Loop set to be launched through prescription, it was important to also improve the Tidepool Web experience.
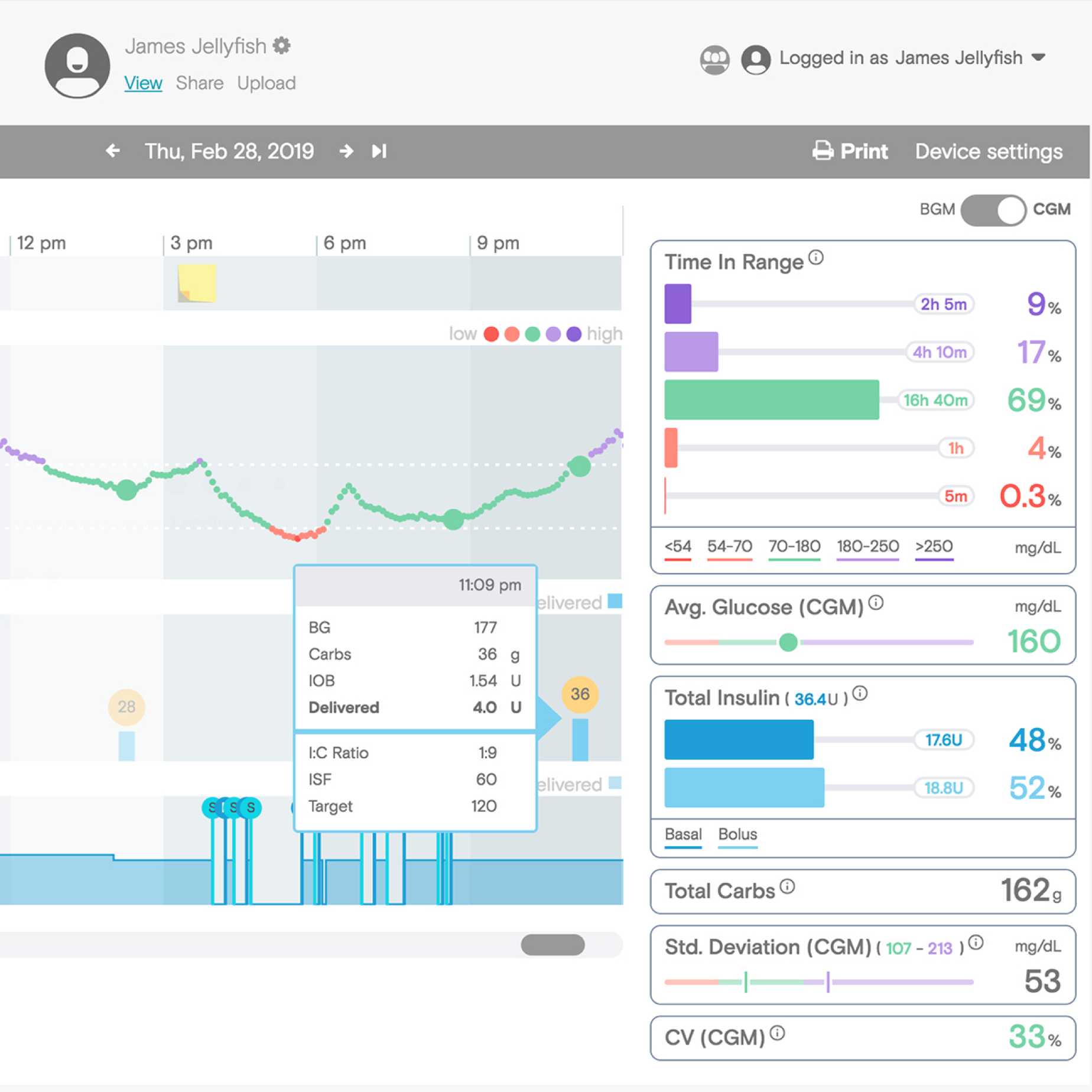
Tidepool Web interface courtesy of Tidepool
Landscape Analysis
To better understand current practices for clinics, I conducted a competitive analysis of other diabetes software companies and also analyzed their information architecture.
.jpg)
Exploratory Interviews
I interviewed six clinicians and generated feature prioritization and design proposals after synthesizing their responses. Common pain points that arose included:
- EHR Integration
- Multiple clinic workspaces
- Pre-visit patient support
- Accounty Security
This research guided the UX design of the clinic interface and is currently under development.
4. Futures Workshops
Type 2 Visioning
Through conversations with Tidepool’s partner organizations, there was an opportunity to expand the product’s scope beyond its original focus on Type 1 diabetes. We began exploring how the system could support people with Type 2 diabetes, a population with different clinical needs, behavioral patterns, and relationships to data and technology.
I helped frame early design questions around these differences, such as varying levels of insulin use, device familiarity, and care team involvement, and led visioning workshops with the design and product teams to consider what future applications of the platform might look like in the Type 2 space.
.png)
I created several blue-sky mockups to show how Tidepool Loop could be expanded for the Type 2 experience for potential device partners.
In Closing
Tidepool Loop was submitted to the FDA in December 2020 and received 510k Clearance in January of 2023. The human factors FDA reviewer commented during a meeting that the HF report I co-authored was one of the best he'd seen and that it'd "made his year." I learned an incredible amount being a part of Tidepool's first 510k submission and am very grateful to the contributions of the diabetes community that made it possible.


.png)
.png)









.jpg)


.png)
